What’s a Fuel Cell?
A fuel cell in a hydrogen car operates a little like a battery, although unlike a battery, a fuel cell does not run down or need recharging. It will produce energy in the form of electricity and heat as long as fuel is supplied. A fuel cell consists of two electrodes sandwiched around an electrolyte. Oxygen passes over one electrode and hydrogen over the other, generating electricity, water and heat.
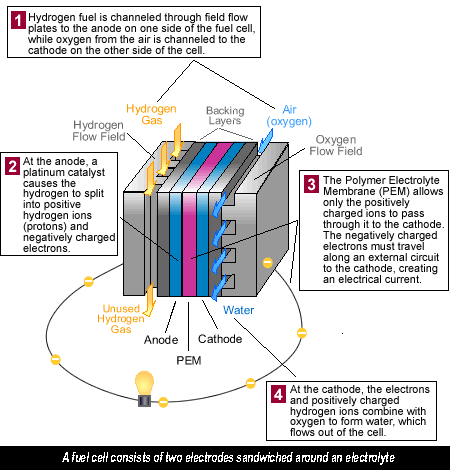
Fuel cells run on hydrogen, the simplest element and most plentiful gas in the universe. Hydrogen is colourless, odourless and tasteless. Each hydrogen molecule has two atoms of hydrogen, which accounts for the H 2 we often see. Hydrogen is the lightest element, with a density of 0.08988 grams per litre at standard pressure, yet it has the highest energy content per unit weight of all the fuels – 52,000 Btu/lb, or three times the energy of a pound of gasoline. Unfortunately, it takes a lot of energy to create hydrogen in order to run a hydrogen car.

