Hydrogen Cars
Hydrogen is never found alone on Earth; it is always combined with other elements such as oxygen and carbon. Hydrogen can be extracted from virtually any hydrogen compound and can be produced from diverse domestic feed stocks using a variety of process technologies. Hydrogen-containing compounds such as fossil fuels, biomass or even water can be a source of hydrogen. Thermochemical processes can be used to produce hydrogen from biomass and from fossil fuels such as coal, natural gas and petroleum. Power generated from sunlight, wind and nuclear sources can be used to produce hydrogen electrolytically. Sunlight alone can also drive photolytic production of hydrogen from water, using advanced photoelectrochemical and photo biological processes. However, hydrogen is not a source of energy, but only a carrier of energy, and hydrogen as a fuel for transport is far from being perfect.
On Board Hydrogen
This is not new technology, and water to gas converters have been used in vehicles for many years. Using a simple device, electricity from the battery is used to separate water (H2O) into a gas known as HHO. HHO is two parts Hydrogen and 1 part Oxygen. HHO burns effectively and produces energy, and is said to increase fuel economy.
Gas4Free is a conversion manual for Onboard Hydrogen systems, and shows how to build and install systems for all types of vehicles.
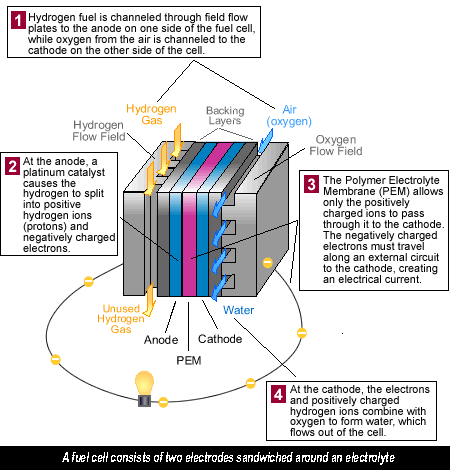
What’s a Fuel Cell? Fuel cells in a hydrogen car operate a little like a battery, although unlike a battery, a fuel cell does not run down or need recharging. It will produce energy in the form of electricity and heat as long as fuel is supplied. A fuel cell consists of two electrodes sandwiched around an electrolyte. Oxygen passes over one electrode and hydrogen over the other, generating electricity, water and heat.
Fuel cells run on hydrogen, the simplest element and most plentiful gas in the universe. Hydrogen is colourless, odourless and tasteless. Each hydrogen molecule has two atoms of hydrogen, which accounts for the H 2 we often see. Hydrogen is the lightest element, with a density of 0.08988 grams per litre at standard pressure, yet it has the highest energy content per unit weight of all the fuels – 52,000 Btu/lb, or three times the energy of a pound of gasoline. Unfortunately, we will see later that it takes a lot of energy to create hydrogen.
Vehicles
Hydrogen can also be used as a fuel directly in an internal combustion engine not much different from the engines used with gasoline. The problem is that while hydrogen supplies three times the energy per pound of gasoline it has only one tenth the density when the hydrogen is in a liquid form and very much less when it is stored as a compressed gas. This means that hydrogen fuel tanks must be large.
The Western Australian Government (Australia) owns three fuel cell buses (see below), which are operated as part of the Transperth public transport system. The buses are part of a limited series of Mercedes-Benz Citaro fuel cell buses being manufactured in Mannheim, Germany by EvoBus for a series of International Trials. The purpose of the trial is to determine the critical technical, environmental, economic, and social factors that need consideration in the introduction of hydrogen fuel cell buses. The Perth trial is also structured to examine what Government and private sector systems are needed to support a hydrogen based energy system.

The German car company BMW, have created the BMW Hydrogen 7. Unlike other hydrogen cars the Hydrogen 7 does not used hydrogen fuel cells, rather it burns liquid hydrogen directly into its modified engine. This hydrogen car is able to run on both gasoline and liquid hydrogen. It delivers amazing horsepower and top speed is not compromised at 228 kph (142 mph) without creating exhaust gas. The car can be driven 27 Kilometres on (17 miles) on 3.7 litres (1 gallon) of hydrogen in the gasoline mode. The downside of this hydrogen car is the estimated cost of about two million dollars (USD).

Efficiency
Hydrogen cars and fuel cells are often promoted as being potentially emission-free if they burn hydrogen, in contrast to currently more common fuels such as methane or natural gas that generate carbon dioxide. However, as mentioned above hydrogen is an energy carrier, not an energy source. This means that hydrogen produced from natural gas has only 50% of the energy of the original natural gas. Hydrogen can also be produced from oil but the energy efficiency is a little worse. If hydrogen is derived from fossil fuel, carbon dioxide is released in all cases.
Electrolysis, which requires electricity, is used to extract hydrogen from water. In the United States in 2005, 72 percent of all electricity generation came from coal and fossil fuels (coal, petroleum, and natural gas), while nuclear electric power contributed 19 percent, and renewable energy resources 9 percent. When hydrogen is produced through electrolysis, the energy comes from these sources. Though the fuel cell itself will only emit heat and water as waste, pollution is produced to make the hydrogen that it runs on. Hydrogen production requires huge amounts of energy to isolate the hydrogen, significant energy to compress it in the tanks, and is only as clean as the energy sources used to produce it.
Storage
Developing safe and reliable hydrogen storage technologies that meet performance and cost requirements is critical to achieving a future hydrogen economy. Hydrogen storage would be needed for both vehicular applications and off-board uses such as for stationary power generation and for hydrogen delivery and refuelling infrastructure. The ability to carry enough hydrogen on-board a vehicle to enable a driving range of greater than 400 kilometres (approx 250 miles), within packaging and cost constraints, is a great challenge. A downside in storing liquid hydrogen, is some of the gas must be allowed to evaporate for safety reasons, meaning that after a couple of weeks, a hydrogen car could lose half of its fuel, even when not being driven.
Cost – A Major Hurdle
So why aren’t fuel cells being installed everywhere there is a need for more power? The primary reason is cost. Fuel cells developed for the space program in the 1960s and 1970s were extremely expensive at around $600,000 per kilowatt and impractical for anything here on Earth. During the past three decades, more practical and affordable designs for stationary power applications have been developed, but progress has been slow. Today, the most widely deployed fuel cells cost about $4,500 per kilowatt; by contrast, a diesel generator costs $800 to $1,500 per kilowatt, and a natural gas turbine can be $400 per kilowatt or even less.
There is an argument that the ‘hydrogen economy’ doesn’t make much sense, as we need to solve an energy problem not an energy carrier problem. Substantiated claims are made that production of hydrogen can consume as much as 75% of the energy produced, leaving only 25%, which is simply not cost effective energy production. Ultimately, Hybrid and Plug-in Hybrid Vehicles are likely to be a much better proposition than hydrogen cars.