
The direct sampling of volcanic gases is very up close and personal, and is ideally suited for long term study of volcanoes. This type of study produces detailed chemical analysis of specific fumaroles and vents. The method is not well suited for monitoring rapidly changing conditions, however, because of the time necessary for completing a laboratory analysis, which can run into days or often weeks. Being so close to an active volcano is often unsafe for scientists, particularly when sampling at or near the potential eruptive vent, and a volcano becomes increasingly restless.
Generally volcanic gases are collected by putting a chemically inert and heat resistant tube into a hot opening. The tube is allowed to heat up until any condensation in the tube has reached equilibrium with the escaping gases, usually about 5 minutes. Then, either a specially-designed evacuated-sample bottle or a flow-through sample bottle is attached to the collection tubing to gather the gases. 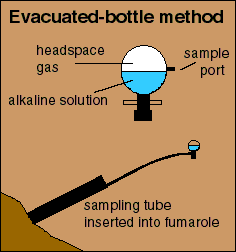 The evacuated-bottle method utilizes a borosilicate glass bottle with a high-vacuum stopcock and sample port. Before arriving at the sampling site, the bottle is partially filled with concentrated aqueous sodium hydroxide, carefully weighed, and evacuated with a vacuum pump. As gases from a fumarole bubble through the alkaline solution, acid gases, such as CO2, H2S, SO2, HCl, and HF dissolve into the liquid.
The evacuated-bottle method utilizes a borosilicate glass bottle with a high-vacuum stopcock and sample port. Before arriving at the sampling site, the bottle is partially filled with concentrated aqueous sodium hydroxide, carefully weighed, and evacuated with a vacuum pump. As gases from a fumarole bubble through the alkaline solution, acid gases, such as CO2, H2S, SO2, HCl, and HF dissolve into the liquid.
The remaining gases, such as N2, O2, H2, CO, and He, collect in the headspace of the bottle. Many litres of fumarolic gas can be collected in a single bottle, because volcanic gas is typically composed mostly of water and condensable acid gases. This method concentrates gases in the solution and headspace, and thereby promotes better analytical precision. The headspace gases are analysed by gas chromatography while the gases in solution are analysed by ion chromatography or traditional wet-chemical methods.
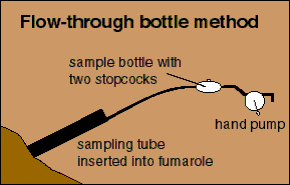 In the flow-through bottle method a glass bottle with a stopcock at each end and a hand-operated pump are attached to the sampling tube. The hand pump flushes out air and draws the fumarole gases into the bottle. Because this collection method is faster, it is used when a complete gas analysis is not necessary, or where field conditions are too hazardous to safely make an evacuated-bottle collection. Sulphur gases are stable for only a short time so this type of sample must be analysed within a few hours of collection.
In the flow-through bottle method a glass bottle with a stopcock at each end and a hand-operated pump are attached to the sampling tube. The hand pump flushes out air and draws the fumarole gases into the bottle. Because this collection method is faster, it is used when a complete gas analysis is not necessary, or where field conditions are too hazardous to safely make an evacuated-bottle collection. Sulphur gases are stable for only a short time so this type of sample must be analysed within a few hours of collection.
Both the Hawaiian Volcano Observatory and the Cascades Volcano Observatory are equipped to analyze gas samples for SO2 and CO2. If a complete and detailed analysis is required, samples are sent to an analytical laboratory at the USGS Western Region Center in Menlo Park, California. The advantage of a detailed analysis is that it provides the necessary information to perform thermodynamic calculations and construct models in order to provide insight into the condition of the magma at depth from which the gases originated.
Information for this page on sampling volcanic gases was sourced from the USGS web site, Direct gas sampling and laboratory analysis.
